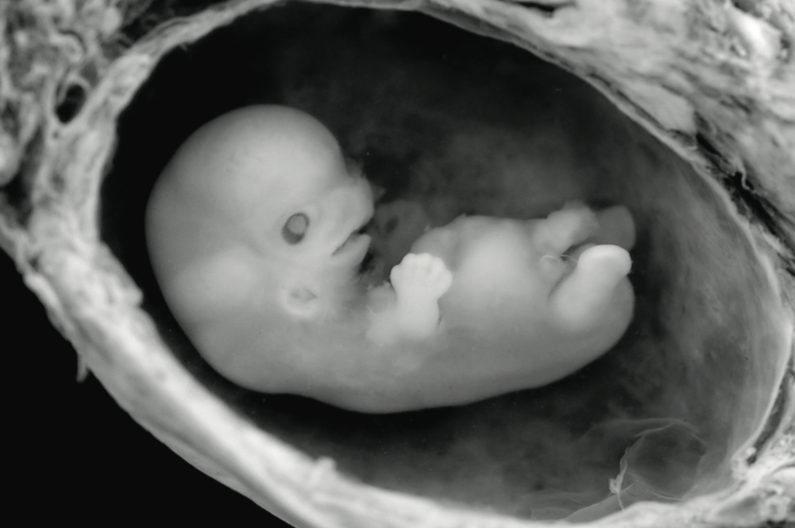
Science fictions and fantasies are quickly becoming facts with CRISPR, a gene-editing technology that is opening up new horizons for the human species. Some dream of turning horses into unicorns, while others would like to normalize humans — eliminating rare gene mutations from our populations. Biologists are considering hacking the genomes of unwanted insects such as mosquitoes, giving them destructive sequences of DNA to propel entire species to extinction.
In October 2015, The New Yorker hosted an open-ended discussion forum about CRISPR, which the magazine billed simply as a “cutting-edge gene technology.” Our discussions about emergent 21st century biotechnology dreams took place against the austere backdrop of the 9/11 Memorial, looking down from the 23rd floor of the new Freedom Tower, the monolith built to replace the Twin Towers. The host was Michael Specter, a veteran biotech reporter with a salt-and-pepper beard, who wryly characterized his profession as one that makes “few mistakes.” In the 1990s, Specter confessed, he was guilty of feeding the media frenzy about Dolly the cloned sheep — adding his own articles to the hysteria about cloning, painting dystopic fantasy scenarios about human clones being grown for organ replacements and writing stories about the leaders of North Korea making copies of themselves.
Hype about CRISPR has already attracted massive investments. As of August 2015, four biotech startup companies focusing on health applications of the technology have raised at least $158-million in venture capital [1]. Vertex Pharmaceuticals made a “$2.6-billion bet” on one of these startups, CRISPR Therapeutics, last November [2]. Other genome engineering technologies, such as zinc fingers and TALENs, have already been used to conduct genetic surgery in clinical trials [3].
The New Yorker panel contributed to the speculative hype about CRISPR. Jennifer Doudna, a professor at U.C. Berkeley and co-founder of Caribou Biosciences, is one of the people credited with developing the technology. Seated at Specter’s right on the stage, she compared CRISPR to a kind of software for the genome. “It is simple to use,” Doudna said, for “people who have basic training in molecular biology.” CRISPR works in all of the living organisms where it has been tested — in humans, plants and animals — and thus has very broad potential applications. “Older technologies,” she said, “were more like hardware … it was like re-wiring your computer every time you wanted to use a new piece of software. Here we have a technology that is easily manipulated and easily changed.” CRISPR is enabling graduate students and postdocs to reengineer the genomes of organisms in a way that is “cheaper, faster, and more accurate.”
Feng Zhang, an assistant professor at MIT and co-founder of Editas Medicine, said, “When I started my lab my students were taking three weeks or three months before they could study neurobiology questions; you had to rewire the ‘computer’ to get it to target a new DNA sequence in the genome. With CRISPR it now just takes students a couple of days.”
“CRISPR originated as a bacterial defense system,” Specter said. “It’s an immune system in the microbes. It was found by mistake in 1987 by some Japanese scientists who were looking for something else. They found a weird group of nucleotides that they couldn’t understand. So they wrote their article, published it in the Journal of Bacteriology, and literally in the last sentence they said: we saw this weird formation and we have no idea what it does.” In 1997, scientists working for Dansk, the yogurt company, were studying bacterial cultures with an eye to helping them stay alive, Specter explained. They saw that all the bacteria surviving viral attacks had one thing in common — they all had CRISPR. The bacteria were using CRISPR to hack up and dismantle the viruses — memorizing, interrogating and cleaving foreign DNA. People began seeing whether they could use this molecular tool for other purposes.
From there things moved very fast. CRISPR, according to Specter, has an enzyme that is like “an X-ACTO knife, a scalpel, something that cuts with precision.” There is another enzyme in CRISPR “that is like a guide, it is an RNA probe, sort of like a fairy.” This guide takes the cutting knife “exactly to where you want it to go in the genome. The idea is that you cut out bad things, you replace them with good things, and we are all happy … Basically it is a genetic GPS system … One of the things that people want to do is change genes—take out the broken stuff, put in better stuff.”
Discussion on the panel mostly centered on the utilitarian possibilities emerging with CRISPR. Panelists described known genetic pathologies that can now be eliminated from the human species. Disease transmission pathways are being disrupted with novel genetic tools. Crops and domesticated animals are being tweaked to make them more productive and more docile.
Speculation about uses and potential abuses of CRISPR also briefly ventured into the realm of whimsical fantasy. Henry Greely, a bioethicist who is a professor of law and director of the Center for Law and the Biosciences at Stanford, said that it was only a matter of time before a Silicon Valley billionaire decided to make a unicorn with CRISPR for his daughter’s birthday. The audience let out a small collective gasp when it was revealed that related science fictions and fantasies have already become scientific fact. Cattle breeders have used CRISPR to make bulls with no horns, taking genes from animals without horns (the products of hundreds of years of selective breeding) and splicing them into lines where horns have yet to be eliminated with conventional techniques. If turning off horns can already be done in cattle, then turning on a horn gene in horses might not be that difficult.

CRISPR can be used to edit the somatic cells of an organism — the cells in an adult’s body — as well as the germ line — the cells destined to become sperm and eggs, passed on to future generations. Kevin Esvelt, an assistant professor at Harvard who describes himself as an “evolutionary sculptor,” reminded the audience that genes passed along to future generations are subject to natural selection. Invoking Darwin, Esvelt said that “natural selection is a power incessantly ready for action. It is immeasurably superior to man’s feeble works, as works of nature are to works of art. What that means in practice is that whenever we tweak a natural organism — and it doesn’t matter if we do it by traditional selective breeding over many gen- erations, or modern genome editing — we reduce its ability to survive and reproduce in the wild. Our edited organisms — whether they are our pets or laboratory organisms — will be eliminated by natural selection if released into the wild. Our changes don’t survive.” In other words, people should not be afraid of CRISPR critters, according to Esvelt, since they will not be able to survive without ongoing care and nurturing.
Novel forms of wildlife created with CRISPR could indeed spread, if one makes different assumptions about population genetics. The neutral theory of molecular evolution assumes that most genetic diversity is caused by the random drift of mutant alleles that are neutral rather than the propagation of efficient genes according to principles of fitness. Motoo Kimura, a Japanese scholar, formulated the neutral theory of evolution in 1968 [4]. Esvelt’s reasoning about natural selection reflects the dominant Anglo-American consensus about neo-Darwinian evolution, which ignores Kimura’s important work. As long as a given edited gene does not kill the organism in question, a CRISPR critter could breed — re-sorting and reassembling genes according to the principles of reproductive biology. The guiding slogan of Sandra Gil- christ, a marine biologist who taught me heterodox theories of population genetics, is “It doesn’t have to be efficient; it has to be sufficient.”
Esvelt is relatively unconcerned about plain old vanilla CRISPR critters. However, he rang a loud cautionary bell in the forum about another technology he is tinkering with: the gene drive. While edits made to an organism’s genome with CRISPR remain subject to standard evolutionary and ecological principles, the gene drive is a new kind of hardware that can be wired into a population of breeding organisms. “Sculpting Evolution,” Kevin Esvelt’s personal website (sculptingevolution.org), has an FAQ section that asks, “What is a gene drive system?” The simplified answer is: “It’s a stretch of DNA that is inherited more frequently than normal. In sexually reproducing organisms, most DNA sequences have a 50% chance of being inherited by each offspring. This is called ‘Mendelian inheritance.’ Gene drive systems manage to rig the game so that they are inherited more frequently — up to 100% of the time” [5].
The genetic processes sound innocuous, but the danger comes with how they might be used. Esvelt knows how to hard wire gene drives into organisms, making sure that destructive DNA quickly spreads through a population. Researchers are already using the gene drive to manipulate malarial mosquitoes. Esvelt said he might try to insert destructive sequences into mosquito genomes to propel entire species to extinction.

While targeting some unloved animals for destruction, Esvelt is also thinking about using the gene drive to manipulate complex ecosystem dynamics. For example, he is working to eliminate Lyme disease, pathogenic bacteria that live in the White-footed Mouse, one of their reservoir hosts in the eastern United States. Esvelt wants to bioengineer White-footed Mice that have antibodies to Lyme disease hardwired into their genome. This kind of intervention would theoretically create a better world where mice, ticks and humans could flourish alongside each other, no longer entangled in a debilitating dance with a bacterial disease. If bioengineering resistant mice proves too difficult, Esvelt plans to focus on the tick. As with the malarial mosquito, Esvelt is considering targeting certain tick species for extinction.
While laying bare his plans, Esvelt explained how the gene drive works. “CRISPR is a scalpel that enables us to cut the DNA where we want and introduce a new sequence to replace it. But, what if instead of just doing that we introduce the genes encoding CRISPR along with it? So then the scalpel itself is now heritable. When an organism mates with a wild counterpart, the offspring inherit one copy of the altered gene and the Crisper scalpel and one copy of the original wild version of the gene. The scalpel then — in the offspring — cuts the wild version and copies over the altered gene and the CRISPR scalpel. So, when that organism [reproduces] all of its children will inherit copies. And if the other copy is wild type, it happens again. By doing this CRISPR enables us to spread an alteration we make in the laboratory through, in principle, through an entire wild population.”
What, Me Worry?
Michael Specter used The New Yorker forum as an opportunity to explore many of the ways CRISPR might go awry. “There are some people in this room who are probably thinking: ‘Really!? We are going to change all of the genes in a living thing and let it fly away and that will be fine. Do we worry about that?’”
Hank Greely, the bioethicist from Stanford, jumped into the conversation, saying “Yes!” There was nervous laughter from the audience. “If you make something faster, cheaper, easier — one hundredfold — it takes over the world,” Greely said. “We worry about using CRISPR to change humans in a way that gets passed down from generation to generation. We worry about using it in humans even if it doesn’t get passed down — if I could play with genes to make my hair turn brown again, I might do it … Athletes might try to improve their abilities.”
“There is one issue here,” Specter added. “When I get a 99 percent score on a test, I’m really smart. If we are 99 percent accurate when we edit cells in trying to get rid of cancer tumors, that’s really bad because we are talking about billions of cells.” Turning to the panelists, Specter asked: “Do we need to be 100 percent accurate and is that really possible?”
Feng Zhang spoke up. CRISPR “is not perfect yet,” he said. “This is one of the reasons why the technology is not mature enough for clinical translation or for germ line editing. It will make what we call ‘off-target’ or ‘side- effect’ modifications.”
Specter recounted a conversation with George Church, a colorful character at Harvard whose lab has done pioneering work with CRISPR. “Gee, you know, we are going to edit stuff, something is going to go wrong,” he remembered saying. “So then we’ll just change it,” Church replied. “Eleven generations from now they will just change it back. What’s the big deal?”
“The ability to undo something if things go wrong is tremendously important,” explained Esvelt, who works with Church at Harvard. “That is certainly the case for a gene drive. You can imagine, if you build a scalpel to spread a particular alteration through the population you can also program a CRISPR scalpel to overwrite that with a new version. We should always, always, always have a lot of organisms in reserve with what we call a ‘reversal drive.’… I am an evolutionary biologist. I am very concerned with the question [of] how these things will evolve over time. Because they will. We need to study them in the laboratory with very, very large populations and over as many generations as possible.”
Writing for the Scientific American blog, Church and Esvelt suggest that the precision of CRISPR would let them put safeguards in place. “Alterations can be reversed by releasing a new drive with an updated version of the change. It’s effectively a slow-motion ‘undo’ button for genome alterations and could work on any type of change” [6].
Gene drives have the potential to run wild, escaping beyond humans’ capacities to care for them and control them. Sarah Franklin contrasts older visions of wildness, which “meant non-domesticated, as in ‘wild geese’ or ‘a wild boar’” with a new sense of the wild emerging within the domains of biotechnology and agriculture. “What might be described as over-domestication or hyper-cultivation turns out now … to produce the other sense of ‘wild,’ as in something dangerous, risky, and out of control — ‘a wild idea’ or ‘a wild night out’” [7].
Wild gene drives, and I would suspect even the “reversal drives,” certainly have the potential to run amok. What if they jumped from species to species? Instead of targeting unloved arthropods, such as ticks and mosquitoes, what if a gene drive infected honeybees — driving these useful animals to the brink of extinction? Instead of targeting cancer cells for destruction within a patient’s body, could the CRISPR scalpel start cutting and pasting genes in a way that generates new kinds of tumors?
Hank Greely, the bioethicist, said experiments with CRISPR are being conducted in a legal vacuum. Right now there are no special regulatory frameworks that govern enterprises to edit the genomes of organisms, he reminded the group. There are no laws that treat bulls whose horns have been turned off with CRISPR any differently from cattle with horns that have been bred out over the course of many generations.
Gene drives are barely understood by the research community, much less by the public. “We are beyond natural selection now,” said Specter. “We are making our own evolutionary choices now, which is scary and exciting.”
Designer CRISPR Babies
After the public event, I invited Kevin Esvelt to grab a bite to eat nearby. We were joined by a small knot of biologists and members of the public who trailed along. Among the group were undergraduates from University College London who have created a “happier” genetically modified microbe. Departing from recent findings about human gut flora, new research suggests that microbes mediate emotions. This group of young tinkerers has created a new kind of E. coli bacteria that enhance the production of serotonin — a molecule associated with well-being and happiness. As we walked past the 9/11 Memorial and talked, Esvelt told me about the field of optogenetics, which involves using lasers to control the behavior of genetically modified animals. Feng Zhang, one of the other panelists, has recently created some optogenetically enhanced silkworms. Before long, Esvelt speculated, bioartists will be able to use silkworms to “print” works of art — programming a pattern in a computer and then directing the worms with lasers.
We grabbed a table at Panini & Co Breads, a sandwich shop on Cedar Street at the corner of Zuccotti Park, once the central camp of Occupy Wall Street. The conversation quickly turned to designer babies and the future of the human species. “I want eyes that glow orange,” said one science enthusiast who had joined us. “That would be cool.”
“People want to become like the characters in Marvel comics,” said one of the other biologists as he sat at the table. “If you look at how muscles and bones actually develop, they won’t properly form unless you have the motor neurons properly firing and stimulating them,” he added. “So you actually can’t CRISPR yourself into the Incredible Hulk. You would still have to lift weight, if only to train the motor neurons to fire those muscles.”
Physical and mental abilities are coded by many different genes. Traits such as the color of one’s skin, eyes and hair are coded by a handful of genes and would be easy to manipulate with CRISPR. But the disability studies scholar Gregory Wolbring warns that consumers should not simply be allowed to create so-called perfect babies. People who are viewed as “unfit” or unhealthy could be eliminated from the population by being “fixed” with gene editing technologies, or even prevented from being born [8].
It is already theoretically possible to look at the entire genome of a fetus and go through the genes one by one. Each gene in the double-stranded DNA helix has two copies: one from the mother, one from the father. Clinicians might soon be able to use CRISPR to decide which copy of a given gene to keep — the version from Mom or the version from Dad. Cut out one and paste in the other. Cut and paste; cut and paste. “Since the genetic information of each individual fetus has such mind-boggling complexity, computer algorithms might be necessary to sort through everything, separating the desirable genes from the undesirable,” said Kevin Esvelt.
“If you just get rid of the negative mutations, that is the biggest low-hanging fruit,” said another biologist who asked not to be named.
“There could be great improvements in physical and mental health if we just remove all of the things that break,” Esvelt elaborated.
“Humans want babies that are exceptional,” the other biologist added, “but the most genetically average human might be the most exceptional, not like superheroes in Marvel comics.”
Gene mutations that are exceptional, that are represented in less than one percent of the population, should be eliminated from the population, according to Esvelt. Rare genes like these, he reasons, are likely to be harmful.
“The flipside is that you could have mutations that you care about,” he continued, “and the reason why they are not fixed in the population is that they are costly in some area we don’t care about. In our ancestral environment some things may have been highly, highly deleterious. Being immune-compromised would have been a death sentence. Now depending on what the benefit is, it might be worth it.”

I described my recent encounter with pieces of Einstein’s brain in Philadelphia’s Mutter Museum. This macabre museum chronicles the diversity of the human species — exhibiting so-called Siamese twins alongside bird-headed dwarfs, a grotesquely enlarged colon next to a collection of plastic toys, nails, buttons and pins that had been found inside people’s intestines. Slides of Einstein’s brain, cross sections 20 microns thick, are presented alongside text giving visitors context for interpretation: “Scientists who have examined his brain have concluded that it is not normal. While Einstein’s brain weighs less than the brain of an average adult male, 2.7 lbs versus 3 lbs, the inferior parietal region of the brain is 15% larger than in an average brain. Some scientists think that the brain lacks an anatomical crevice called the Sylvian fissure.”
Esvelt did not directly respond to my point about “exceptional” people like Einstein, members of our species who might be outliers, with genetic mutations not widely represented in the population. He simply said that characteristics such as intelligence are difficult to link to genetics. Long legacies of eugenics lingered in the background as we talked. Anthropologist Rayna Rapp has illustrated the eugenic consequences of testing fetuses. Certain people, such as those with Down syndrome and other genetic diseases detectable with amniocentesis, are being systematically eliminated from the population. As Rapp documents, expectant parents whose unborn children test positive for innumerable conditions are usually guided toward abortion by doctors, nurses and extended networks of friends and family [9].
Kevin Esvelt is very concerned about the impacts of emergent technologies on the neurodiversity of the human species. “Specifically, if you give parents the option of zero autism risk in exchange for a certain loss of creativity or unusual talents, I expect almost all of them would take it — to the detriment of humanity,” he said. “I don’t want to see population genetics and medical studies to find genes to be targeted with CRISPR.” Instead, he thinks genetic diseases should be eliminated with existing clinical technologies. It is already possible for couples to use in vitro fertilization (IVF) to fertilize 35 or 40 embryos, pull a cell off of each, have them sequenced and then select the best fetus for implantation. “CRISPR is not safe enough now and by the time it is the technology will likely be superseded by better options,” he predicted. “IVF preimplantation diagnosis is available now, and eventually variants of it will be markedly superior to CRISPR once available.” Esvelt said it would cost about $500-billion, according to his back-of-the-envelope calculations, to do IVF and genetic screening for all the new babies in the United States every year. Israel’s national health care system is already funding large-scale preimplantation genetic diagnosis. Any Israeli citizen can now fertilize multiple embryos with IVF and then choose to implant the one that seems to have the best genetic health [10].
The United States should be doing something similar, according to Esvelt. “Because it will be available abroad, the wealthy will be able to access the technology no matter what,” he said. “Keeping the playing field from tilting even more is a matter of basic moral fairness. It’s also highly likely to be a tremendously good investment from the perspective of the country as a whole, and would likely be exceedingly important for national security if any other country embarks on a similar course, as China evidently is strongly considering.” Esvelt would also like to see a massive study of population genetics to gather background on genes that should be targeted for editing. “The fastest way to do this would be to get the Department of Defense to sequence all of the active duty members of the military, since they already have performance and health data for everyone who is enlisted,” he elaborated.
“With the rise of wearable tech, that will be the other massive source of health data,” the biologist who preferred to remain unnamed chimed in. “It would be great if we could actually get the electronic health care records to interact with the wearable tech.
“If you are creating designer CRISPR babies you would want to engineer something that allows you to readily update all of the cells in the body,” he continued. “You don’t want it to be a gene drive — because you don’t want the cells to divide — you want it to be more like a back-door channel for viruses that have a particular molecular signature. You would want it to be an unnatural molecular signature so that no natural virus could access it.”
Separating natural, cultural and technical agency is not always easy. The deadly H1N1 strain of influenza emerged as a result of factory farming — a single base-pair mutation in the virus suddenly jumped among animals living in crowded conditions, leaping from body to body and then across species lines [11]. Opening up a back door into our genome has the potential to let new kinds of wild life wreak havoc inside.
Esvelt thinks it theoretically would be possible to establish a backdoor channel, an “unnatural molecular signature,” but such a signal is still “quite far in the future.” He readily admitted that a back door into the human genome would make people vulnerable to bioterrorists and hackers. “The same deal with wearable tech,” he said.
“Or the onboard computer in your car,” I added.
Esvelt told us about his recent experience at a Biological Weapons Convention symposium. “A gene drive, because it ignores national boundaries, is a potential bioweapon under all circumstances,” he said.
The biologist who was not willing to be named interrupted: “The scientists who go to these kinds of meetings are generally not at the forefront of the field and do not generally have a good idea about what is going on. They have a very good idea about what diplomats actually want people to do. The diplomats want to look like they know what is going on, but they want to do something about it in a way that has very little political cost.”

Article one of the Bioweapons Convention allows researchers to develop things that could be used as bioweapons as long as they are for peaceful purposes. “I strongly believe that we have a moral obligation to be transparent when conducting research that could impact others if something were to go wrong in the laboratory,” Esvelt said. “In addition to being the right thing to do, transparency and community direction of technology development will increase the chances that the technology will be used wisely.” Esvelt also thinks that transparency and good community norms, rather than binding laws, are all that are needed to keep gene drives from running amok in ecological communities. When some of his colleagues recently attended the 2014 U.N. Convention for Biodiversity conference in South Korea, he encouraged them to argue for “transparency” in research before gene drives are released into the wild.
As the conversation turned to ecology, Esvelt said that saving some endangered species in the wild might not be the best strategy. “I am strongly opposed to spending a lot of money saving charismatic megafauna when we could spend a comparative pittance to biobank samples and use the difference for habitat preservation to benefit many more species,” he asserted. He added that ecosystems are not optimized for biodiversity; they could be tweaked if biodiversity is the goal.
“If the rhino goes extinct it doesn’t matter, because we will be able to print one in a few decades,” added another biologist.
The conversation turned to George Church, the colorful geneticist who Kevin Esvelt works with at Harvard. Church garnered headlines in 2015 when he spliced wooly mammoth genes — coding for small ears, subcutaneous fat, hair length and color — into living elephant skin cells. “If the researchers can get these hybrid mammoth-elephants to survive,” reported Tanya Lewis for livescience.com, “they hope to engineer an elephant that can survive in cold climates, where it should face fewer threats from humans” [12].
Life in the age of biotechnology is increasingly difficult to track. Calls for transparency notwithstanding, it is difficult to account for all of the ways that genes in diverse kinds of critters are being edited and modified. The human species is experimenting with new possible futures as Daft Punk lyrics play in the background:
We are human, after all. Much in common, after all.
Work it, make it, do it [makes us] harder, better, faster, stronger.
Notes
1. Amy Maxmen, “The Genesis Engine,” Wired, August 2015, 62.
2. http://www.fool.com/investing/general/ 2015/11/07/vertex-pharmaceuticals-26-billion-bet-on-crispr.aspx
3. Sara Reardon, “Gene-Editing Method Tackles HIV in First Clinical Test,” Nature News, March 5, 2014, http://www.nature.com/news/gene-editing-method-tackles-hiv-in-first-clinical- test-1.14813.
4. Motoo Kimura, “Evolutionary Rate at the Molecular Level,” Nature 217 (1968): 624–626.
5. http://www.sculptingevolution.org/gene drives/genedrivefaq
6. http://blogs.scientificamerican.com/guest-blog/gene-drives-and-crispr-could-revolutionize-ecosystem-management/.
7. Sarah Franklin, “Ethical Biocapital,” in Remaking Life and Death: Toward an Anthropology of the Biosciences, eds. Sarah Franklin and Margaret Lock (Santa Fe: School of American Research Press, 2003).
8. Natalie Ball and Gregor Wolbring, “Portrayals of and Arguments Around Different Eugenic Practices: Past and Present. International Journal of Disability, Community, and Rehabilitation 12, no. 2 (2013), http://www.ijdcr.ca/VOL12_02/articles/ball.shtml.
9. Rayna Rapp, Testing Women, Testing the Fetus: The Social Impact of Amniocentesis in America (New York: Routledge, 1999).
10. Ephrat Levy-Lahad, Human Gene-Editing International Summit, presentation, “International Perspectives,” December 3, 2015; also see Teman, Elly. 2010. Birthing a Mother. Berkeley: University of California Press; Nahman, Michal. “Materializing Israeliness: Difference and Mixture in Transnational Ova Donation,” Science as Culture 15 , no. 3 (2006): 199–213.
11. Celia Lowe, “Viral Clouds: Becoming H5N1 in Indonesia,” Cultural Anthropology, 25, no. 4 (2010): 625–649.
12. http://www.livescience.com/50275-bringing-back-woolly-mammoth-dna.html.
Eben Kirksey is Princeton University’s 2015–2016 Barron Visiting Professor in the Environment and Humanities and a permanent faculty member in the Environmental Humanities program at the University of New South Wales, Australia. Since completing his doctorate at U.C. Santa Cruz in 2008, he has published two books and one edited collection with Duke University Press: Freedom in Entangled Worlds (2012), The Multispecies Salon (2014), and Emergent Ecologies (2015). In 2010, he co-edited a special issue of Cultural Anthropology with Stefan Helmreich, where they coined the phrase “multispecies ethnography” to characterize novel approaches for studying contact zones where lines separating nature from culture have broken down.

